The Organisation
EUROPEAN MEDICINES VERIFICATION SYSTEM (EMVS)
The European Parliament and Council released a Directive on Falsified Medicine. It aims at improving patient safety by mandating the Marketing Authorisation Holders and manufacturers to put a system in place that is preventing falsified medicines from entering the legal supply chain, the European Medicines Verification System. The European Medicines Verification System (EMVS) should guarantee medicines authenticity by an end-to-end verification.

The Antidote
EMVS REQUIREMENTS
In essence, manufacturers will be required to print a Data Matrix code, which incorporates a unique identifier (UI) and apply an anti-tampering device on the outer packaging of all medicines for each individual sales package.
At the point of dispense the medicine will be scanned, checked and verified for authenticity against a national (or supranational) repository. If the UI on the pack matches the information in the repository, the pack is decommissioned and supplied to the patient. Otherwise, if there is a warning related to this pack, then the system will highlight this as an exceptional event and the package will not be supplied to the patient. An investigation needs to determine whether the pack has been falsified or not.
What is a Medicines Verification System?
A medicines verification system, particularly in the context of the European Medicines Verification System (EMVS), is a digital infrastructure designed to enhance the security of the pharmaceutical supply chain. It aims to protect patients by ensuring that all medicines sold are authentic and safe to use. The system enables the verification of product authenticity at any point in the supply chain, from the manufacturer to the pharmacy, through unique identifiers and security features on medicine packaging.
Benefits of Custom Software Solutions for Medicines Verification Systems
1. Enhanced Patient Safety
Custom software solutions can significantly improve patient safety by ensuring that only authenticated and verified medicines reach consumers. By integrating advanced verification mechanisms, these solutions can detect and prevent the circulation of falsified medicines within the supply chain.
2. Improved Supply Chain Transparency
A custom medicines verification system offers end-to-end visibility of the drug supply chain. It allows stakeholders to track the movement of medicines from production to distribution, ensuring traceability and transparency at every step. This not only aids in authentication but also helps in managing recalls more efficiently if necessary.
3. Compliance with Regulatory Requirements
Custom software solutions can be designed to comply with the specific regulatory requirements outlined by the European Parliament and Council, including data protection and privacy regulations. This ensures that pharmaceutical companies and other stakeholders can meet their legal obligations while protecting patient privacy.
4. Real-time Data Access and Reporting
Such systems can provide real-time access to data regarding the verification status of medicines. This capability is crucial for making informed decisions quickly, especially in situations where the authenticity of a medicine is in question. Additionally, custom solutions can generate reports and analytics, helping stakeholders understand trends related to falsified medicines and improve their preventive strategies.
5. Efficient Recall Management
In the event of a medicine recall, a custom verification system can quickly identify and trace the affected batches, facilitating a swift response to remove them from the supply chain. This not only protects patients but also minimizes the financial and reputational impact on the involved companies.
6. Integration with Existing Systems
Custom software can be designed to integrate seamlessly with existing systems used by pharmacies, distributors, and manufacturers. This ensures a smooth workflow and enables the easy adoption of the verification system across the entire supply chain.
7. Scalability and Adaptability
As the pharmaceutical industry evolves, custom medicines verification software can be scaled and adapted to meet new challenges, whether they are regulatory changes, new types of falsified medicines, or advancements in technology. This ensures long-term viability and effectiveness of the system.
How It Benefits All Stakeholders
- Pharmaceutical Manufacturers can ensure the integrity of their products throughout the supply chain, protecting their brand and patient trust.
- Distributors and Pharmacies benefit from a streamlined verification process, enhancing their ability to guarantee the authenticity of the medicines they sell.
- Regulatory Bodies gain a robust tool for enforcing compliance with anti-falsification regulations, enhancing overall public health safety.
- Patients receive assurance regarding the safety and authenticity of their medications, reducing the risk of adverse effects from falsified medicines.
- Healthcare Professionals can prescribe and dispense medicines with confidence in their origin and integrity, ensuring the effectiveness of treatments.
What is Cloud native software?
Cloud-native software is designed for cloud computing, focusing on scalability, elasticity, and resilience. It utilizes microservices architecture, allowing for independent service deployment and communication via APIs, enhancing application flexibility and scalability. Containers and orchestration tools like Kubernetes ensure consistent deployment across environments and facilitate resource optimization. DevOps and continuous delivery principles support rapid, reliable development cycles. Cloud-native applications automatically scale based on demand, are built to handle failures gracefully, and use Infrastructure as Code (IaC) for efficient cloud management. API-based communication further enables independent service development and deployment. These features allow cloud-native software to leverage cloud computing's full potential, offering businesses the agility, efficiency, and scalability needed in the digital age.
The Challenge
In response to the European Parliament and Council's Directive on Falsified Medicine, Assemblysoft was tasked with working as an extended team in developing a critical component of the European Medicines Verification System (EMVS). Our solution aimed to ensure the authenticity of medicines and safeguard the supply chain against falsified products.
After some initial prototyping and an attempt at using the Windows Workflow Foundation to provide the engine capabilities, a number of requirements could not be met so a bespoke solution would need to be developed.

If you would like some assistance with .NET MAUI | Azure | Azure DevOps Services | Blazor Development then please get in touch, we would be glad to help.
Additionally an instrumentation solution for auditing and logging across the entire service architecture needed to be developed.
- Technical Requirements: Developing a system capable of scanning, verifying, and authenticating medicines against a national or supranational repository.
- Compliance with Directive: Ensuring the solution met all regulatory requirements outlined in the Directive on Falsified Medicine.
- Complex System Integration: Integrating the solution within the broader European healthcare infrastructure.
The Solution
The solution made extensive use of Azure Service Bus, Topics and Queues, Worker roles and a bespoke Workflow Engine along with semantic logging.
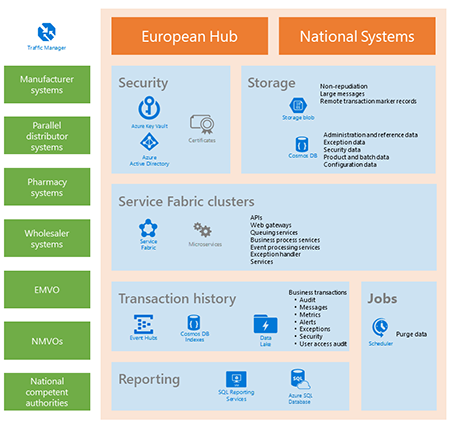
The Workflow Engine
Assemblysoft worked alongside the internal development team and Microsoft consultants to design and build a bespoke Workflow Engine that would run in Microsoft Azure. Having previous experience developing with the Windows Workflow Foundation, we were able to model and apply some best practices to the new engine.
The engine was dynamic and fluid in nature to cater for the amount of different paths into the system and dynamic decisions that would be required based on the characteristics of each request. It proved a big hit with the developers that worked against the design of the coded workflows with 6 sub teams developing individual complex workflows using the model workflow developed to demonstrate typical use-cases.
- Advanced Technology Use: Utilising Azure Service Bus, Topics, and Queues, along with Worker roles, we created a robust infrastructure for the EMVS.
- Bespoke Workflow Engine: After initial prototyping, it was clear that a custom-built workflow engine was necessary to meet the unique requirements of the EMVS.
- Semantic Logging and Auditing: Developing an instrumentation solution for comprehensive auditing and logging across the entire service architecture, ensuring traceability and compliance.
The Instrumentation
Once the core workflow engine development was complete, we turned our attention to the auditing and logging. We utilised a Semantic Logging solution based on the enterprise nature that enabled us to provide custom event sources and schematic data to model the different layers in the application.
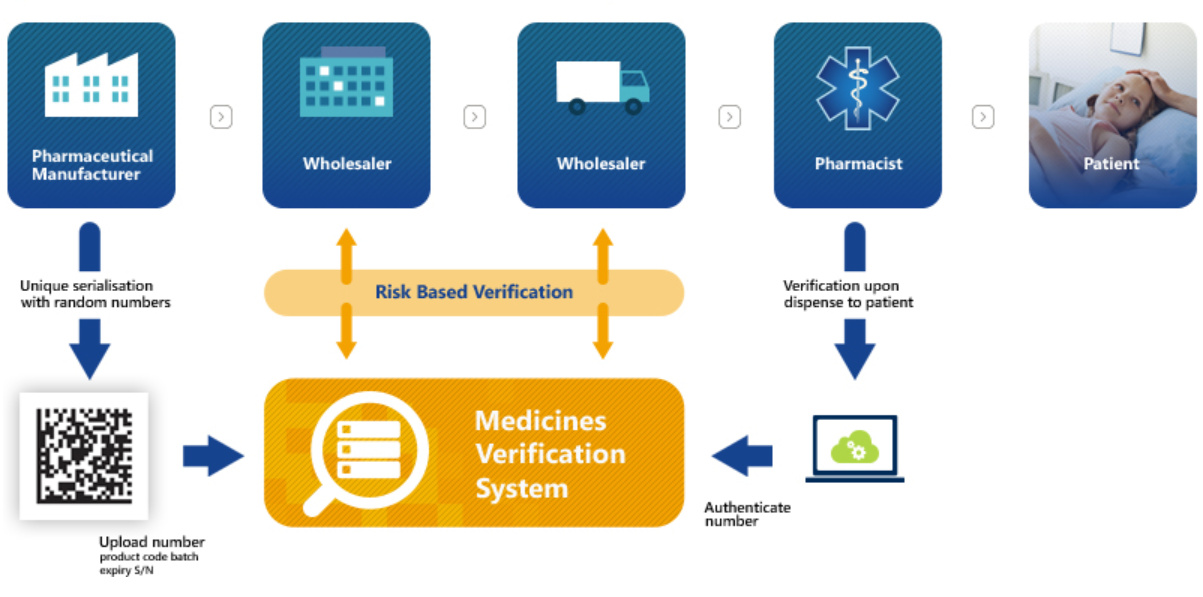
EMVS Requirements Compliance
Our solution meticulously adhered to the EMVS requirements:
- Data Matrix Code Implementation: Ensuring each medicine package was equipped with a unique identifier (UI) within a Data Matrix code.
- Anti-Tampering Mechanism: Incorporating an anti-tampering device on medicine packaging for additional security.
- Verification and Decommissioning Process: Enabling the scanning and verification of medicines at the point of dispense, with a system to decommission or flag exceptional events.
The Results
The bespoke solution delivered significant outcomes:
- Enhanced Medicine Safety: Our system played a crucial role in preventing falsified medicines from entering the legal supply chain.
- Compliance with European Directive: The solution fully complied with the stringent requirements of the European Directive on Falsified Medicine.
- Reliable Medicine Verification: Provided a reliable, end-to-end verification process for medicine authenticity.
Business Benefits
The project offered substantial business benefits:
- Strengthened Public Health Safety: By ensuring the authenticity of medicines, the solution directly contributed to public health safety in Europe.
- Technical Leadership: The development of a custom solution demonstrated Assemblysoft's capability to handle complex, high-stakes projects.
- Compliance and Reliability: Our adherence to regulatory standards and robust solution reinforced our reputation for compliance and reliability in the healthcare sector.
ADDED VALUE
- Delivery : We delivered on time
- Flexibility : The workflow engine covered all the use cases
- Performance: Tests conducted with several billion records
- Cross cutting and consistent Logging and Auditing insights across all teams and application services
Assemblysoft's development of a bespoke solution for the European Medicines Verification System showcases our commitment to public health safety and our ability to deliver complex, regulatory-compliant technological solutions. Our innovative approach to the challenges presented by the European Directive on Falsified Medicine not only safeguarded the medicine supply chain but also positioned us as a key player in the healthcare technology sector.
At Assemblysoft we specialise in Custom Software Development tailored to your requirements. We can onboard and add value to your business rapidly. We are an experienced Full-stack development team able to provide specific technical expertise or manage your project requirements end to end. We specialise in the Microsoft cloud and .NET Solutions and Services. Our developers are Microsoft Certified. We have real-world experience developing .NET applications and Azure Services for a large array of business domains. If you would like some assistance with Azure | Azure DevOps Services | Blazor Development | .NET MAUI Development or in need of custom software development, from an experienced development team in the United Kingdom, then please get in touch, we would love to add immediate value to your business.

Assemblysoft - Your Safe Pair of Hands
